Identification of Two Imidazole Binding Sites and Key Residues for Substrate Specificity in Human Primary Amine Oxidase Aoc3.
Elovaara, H., Kidron, H., Parkash, V., Nymalm, Y., Bligt, E., Ollikka, P., Smith, D.J., Pihlavisto, M., Salmi, M., Jalkanen, S., Salminen, T.A.(2011) Biochemistry 50: 5507
- PubMed: 21585208
- DOI: https://doi.org/10.1021/bi200117z
- Primary Citation of Related Structures:
2Y73, 2Y74 - PubMed Abstract:
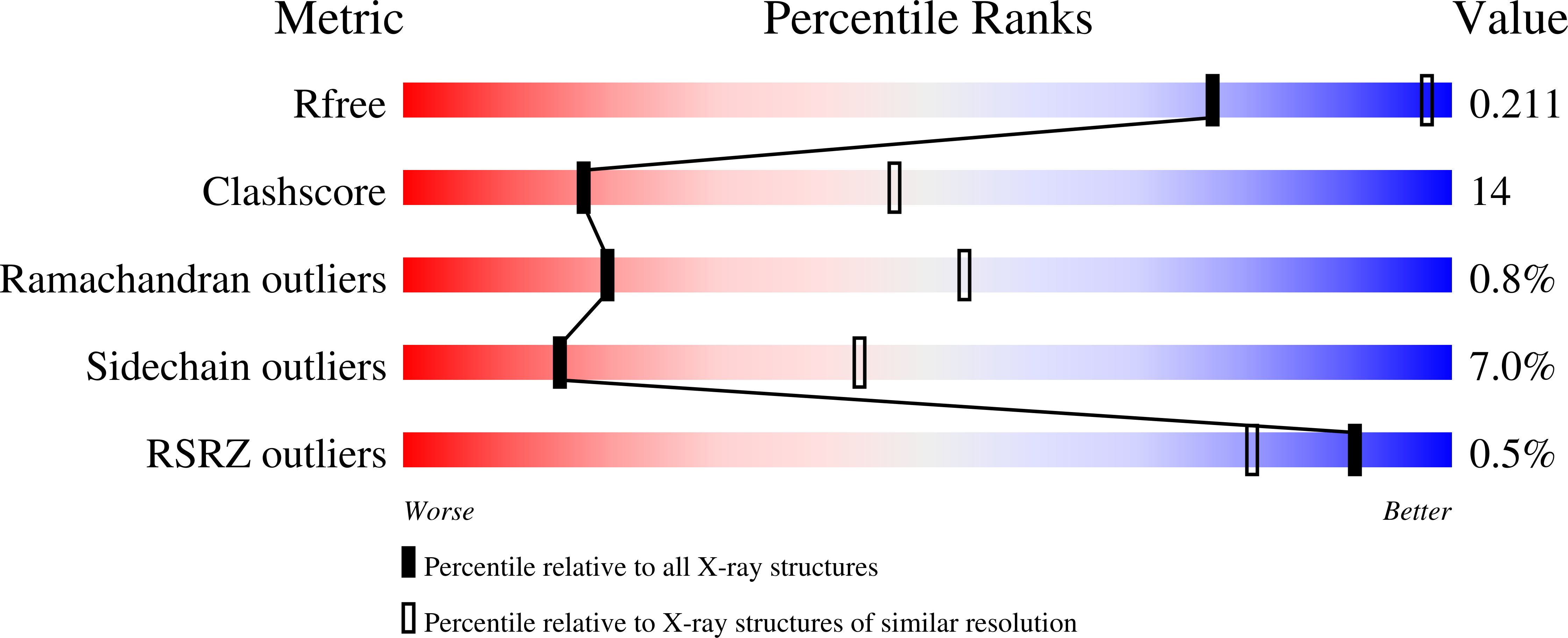
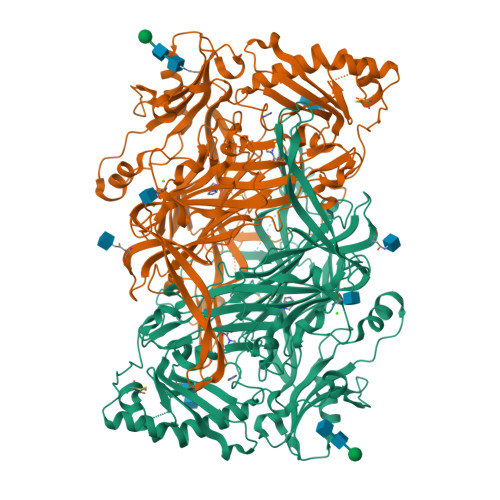
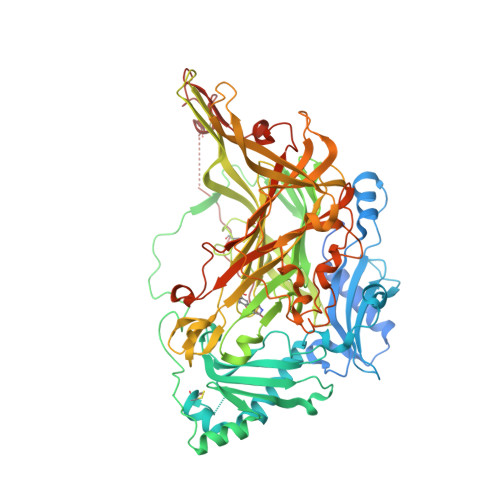
Human membrane primary amine oxidase (hAOC3; also known as vascular adhesion protein-1, VAP-1) is expressed upon inflammation in most tissues, where its enzymatic activity plays a crucial role in leukocyte trafficking. We have determined two new structures of a soluble, proteolytically cleaved form of hAOC3 (sAOC3), which was extracted from human plasma. In the 2.6 Å sAOC3 structure, an imidazole molecule is hydrogen bonded to the topaquinone (TPQ) cofactor, which is in an inactive on-copper conformation, while in the 2.95 Å structure, an imidazole molecule is covalently bound to the active off-copper conformation of TPQ. A second imidazole bound by Tyr394 and Thr212 was identified in the substrate channel. We furthermore demonstrated that imidazole has an inhibitory role at high concentrations used in crystallization. A triple mutant (Met211Val/Tyr394Asn/Leu469Gly) of hAOC3 was previously reported to change substrate preferences toward those of hAOC2, another human copper-containing monoamine oxidase. We now mutated these three residues and Thr212 individually to study their distinct role in the substrate specificity of hAOC3. Using enzyme activity assays, the effect of the four single mutations was tested with four different substrates (methylamine, benzylamine, 2-phenylethylamine, and p-tyramine), and their binding modes were predicted by docking studies. As a result, Met211 and Leu469 were shown to be key residues for substrate specificity. The native structures of sAOC3 and the mutational data presented in this study will aid the design of hAOC3 specific inhibitors.
Organizational Affiliation:
Medicity Research Laboratory, University of Turku, FI-20520 Turku, Finland.